|
| A tree? |
 |
| A vibrant reef? |
 |
| One of those tiny humans your friends are always talking about? |
 |
| Refreshing. |
Wow. H2O sounds pretty sweet, in a flavorless sort of way. No wonder everyone gets so excited when NASA releases another report with evidence of water on Mars.
 |
| As sarcastic as those reports might be. |
It turns out there are several that people much smarter than I have bandied about. One that could exist in our own solar system is methane. On Titan, one of Saturn's moons, it is so cold that small hydrocarbons like methane and ethane are liquid. There are vast oceans and rivers of the stuff, and when it rains, you bet your bottom it's raining men. I mean, methane.
 |
| Artist renderings are the best renderings. |
Of course, it is quite chilly on Titan (-179°C, or -290°F, whichever is your poison), so things would probably happen much slower than they do here on Earth. Another option is formamide, which has a large range of temperatures in which it is liquid and can do things like help synthesize DNA bases (a comforting fact for us Earthlings). More promising still is ammonia, which is liquid in about the same temperature range as water (-107°F to 204°F), as long as you crank up the atmospheric pressure to 60 times what we're used to. It's also great at dissolving lots of different things, which is handy if you'd like to synthesize lots of different things.
Needless to say, we'll really have to think outside the box and keep our minds open to even find life of this sort--by necessity, it would be completely different from what we're used to. So how would we find these lifeforms, if indeed they do exist?
Let us harken back to middle school science class, or some similarly far away time, wherein we learned of:
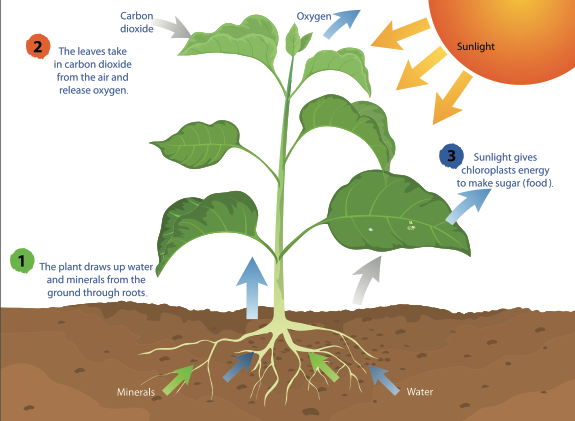
Our first step is to conceive a hypothesis! Now, we know that any life form, big or small, watery or methaney, must 1) consume things, 2) convert these things to other things to obtain energy, and 3) excrete the byproducts of conversion. For example, plants consume sunlight, water, and carbon dioxide to produce glucose, and they excrete oxygen.
At that point, we have hypotheses to test. We could put forth that an organism can consume hydrogen, acetylene, and ethane for energy, and exhale methane as a waste product. Now we mosey on over to Titan and we start looking for the disappearance of hydrogen, acetylene, and ethane, combined with a mysterious increase in methane. Is it life? Maybe not. But from here we can make further assumptions and test them, and so on and so forth.
And you know what's funny? It does seem like there is some "unkown process" consuming--you guessed it--hydrogen, acetylene, and ethane on the surface of Titan....
Reference for that last zinger: Stevenson J, Lunine J, & Clancy P (2015). Membrane alternatives in worlds without oxygen: Creation of an azotosome. Science Advances 1(1):e1400067. doi: 10.1126/sciadv.1400067