Holy crap. If you want to skip straight to the sad parts, check out around 4:20. I couldn't find the video to post it here, but somewhere out there is a video of starfish literally walking themselves apart; two arms going in opposite directions, then *boop* three-armed starfish! Awful.
Obviously, that kind of sucks for starfish, but who really cares? They're not pretty like this lovely critter:
 |
| Pictured: a male violet-crowned woodnymph. Thanks for the photo, Joseph C Boone of Wikipedia land! |
Luckily, you don't need brains to eat, and these dudes eat a lot. They are in many ecosystems what is called a keystone species: a species whose effect on its ecosystem is disproportionate to its population size in that ecosystem. Starfish eat shellfish like sea urchins and mussels, keeping their population in check. If you take starfish out of the equation, the shellfish population explodes. This throws the ecosystem out of balance--suddenly you have a plethora of clams roving around, eating all the food and taking all the nice rocks for themselves. But wait, there's more!
Getting rid of the starfish also destroys a food source for several predators: sharks, rays, and sea anemones. Sharks in particular could just eat more regulat, fish-shaped fish, but that would cause the fish, but that would cause the fish population to plummet and the price of tuna would go up and next thing you know Chicken of the Sea costs $20 a can. It's not a great scenario.
 |
| Pictured: not worth it. |
The cause of SSWS has been a mystery since it was first observed in January of this year, but this month research groups at Cornell (among other places) showed some pretty decent evidence that the condition is caused by a kind of virus called a densovirus. The researchers ground up some diseased sea stars and filtered everything larger than a virus out of the sea star puree (mmmm, tasty), then infected healthy starfish with this virus soup. Within a couple weeks, most of the healthy starfish started showing symptoms of SSWS.
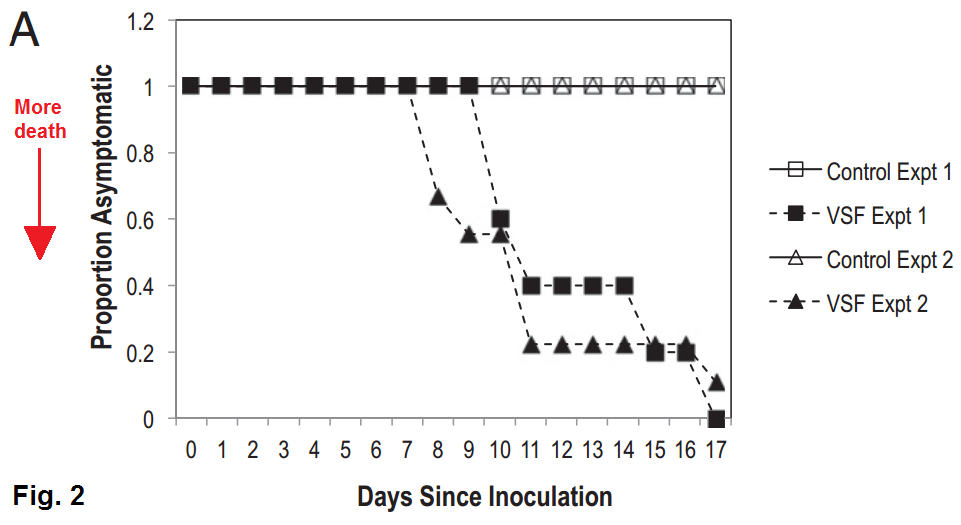 |
| The control is boiled starfish puree. From Hewson et al. 2014, PNAS |
 |
| From Hewson et al. 2014, PNAS |
 |
| I'm poor, so you'll have to deal with the watermark. |
Pictures?
I realized that when I link to a picture here, you can just click on it to get to the original source, so I've decided to stop including the links down here. It only took me three blog posts to figure that out!